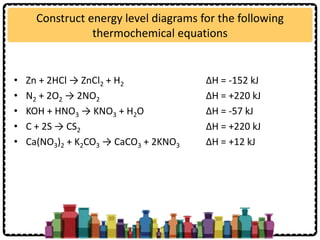
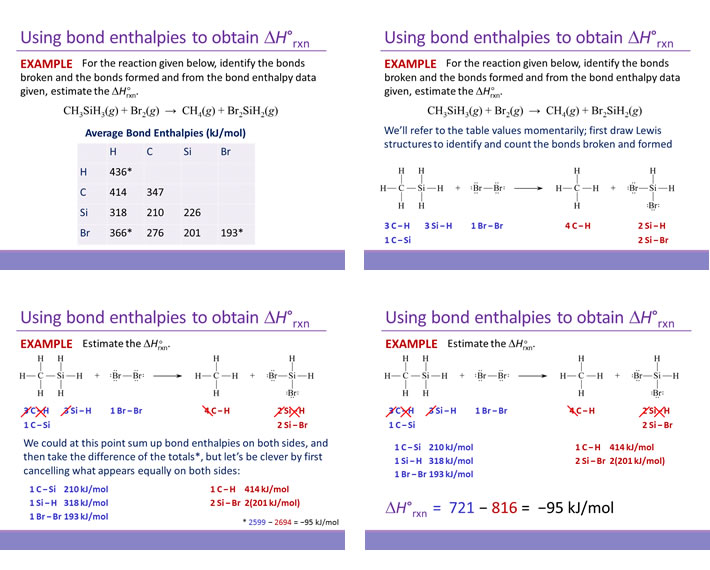
SOLVED: Check Your Understanding: Use the following thermochemical equations to find the heat of formation of diamond: C(diamond) + O2(g) -> CO2(g) ΔH° = -39544 kJ 2 CO(g) + O2(g) -> 2
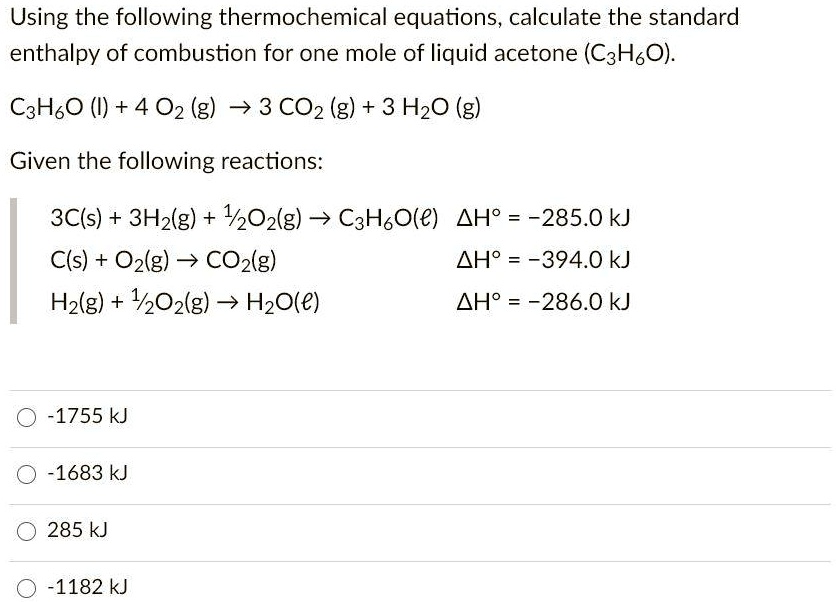
SOLVED: Using the following thermochemical equations, calculate the standard enthalpy of combustion for one mole of liquid acetone (C2H6O). C2H6O (I) + 4 O2 (g) â†' 2 CO2 (g) + 3 H2O (

Unit Thermochemistry Thermochemical Equations Worksheet 3 Answers Form - Fill Out and Sign Printable PDF Template | signNow